Respiratory virus panel, PCR-13532
This test is not available to order at Allina Health. This testing is restricted to Affiliates and outreach client patients only.
Test info
Human Metapneumovirus
Metapneumovirus
RVP
Assay detects influenza A, influenza B, RSV A, RSV B, parainfluenza 1, parainfluenza 2, parainfluenza 3, rhinovirus, metapneumovirus, and adenovirus. Assay also subtypes influenza A virus (H1, 2009 H1, and H3) and adenovirus (B/E and C).
Multiplexed detection of respiratory viruses using the eSensor(R) RVP PCR assay (GenMark).
PCR testing cannot be added on to a sample that has been opened and/or used for other testing. If the sample has already been used for testing, a new specimen will need to be collected.
Specimen
Freeze
To avoid delays in turnaround time when requesting multiple tests on frozen samples, submit separate frozen swpecimens for each test requested.
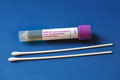
Copan UTM-RT transport (purple cap)
Sterile container
Sterile container
Frozen (preferred) - 28 days
Refrigerated - 7 days
Ambient - NO
- Quantity not sufficient for analysis
- Specimen grossly contaminated
- Leaking or broken tube
- Specimen received at room temperature
Performance
Multiplex polymerase chain reaction (PCR)
Clinical and Interpretive info
Negative