COVID-19 Mayo 2 [Non-Allina sites only]
COVID-19 Mayo 2 [Non-Allina sites only]-14334
Visit the CDC or MDH website for more information as it becomes available.
Contact MDH at (651) 201-5414 or (877) 676-5414 for consultation and questions.
2019 Novel Coronavirus (COVID-19), NAA
Coronavirus
CoV
COVID-19
nCoV
SARS-CoV-2
Detection of coronavirus disease 2019 (COVID-19) illness due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Recommended only for patients who meet current clinical and/or epidemiologic criteria defined by federal, state, or local public health directives: CDC Overview of Testing for SARS-CoV-2
Not for order by Allina Health sites.
There are no food or drink restrictions prior to collection of nasopharyngeal or oropharyngeal samples for COVID-19 testing.
COVID-19/Coronavirus sample collection kit
Kits have been assembled by the Central Lab to include an NP or OP swab and transport media. Note that the contents of the kits may vary as supply inventory is fluctuating.
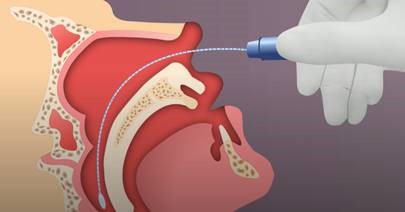
- Use the mini-tip NP swab included in the COVID-19 test kit.
- Tip the patient’s head back.
- Gently insert the NP swab into the nostril parallel to the palate (not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx.
- If any resistance is met in the passageways, do not force the swab; back off and try reinserting it at a different angle, closer to the floor of the nasal canal, or try the other nostril.
- Gently rub and roll the swab for 10-15 seconds while the swab is in contact with the nasopharyngeal wall.
- The CDC recommends leaving the swab in place for several seconds to absorb secretions.
- Slowly remove the swab and place in the transport medium.
- Break the swab shaft so that it fits into the medium container and recap tightly.
- Label the specimen appropriately. Document the source “NP” on the label.
- Request that the patient reapply their mask.
New England Journal of Medicine video on NP swab collection: Nasopharyngeal Swab Collection Video
COVID-19 sample collection kit
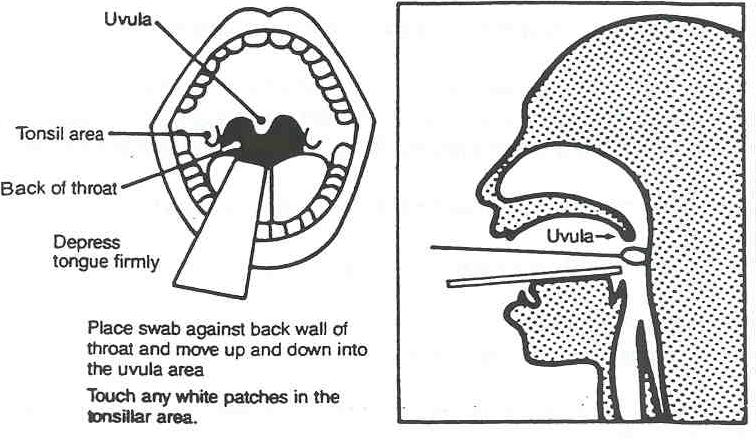
Oropharyngeal (OP) swab:
- Ask the patient to tilt head back, open mouth wide, breathe deeply, and say "ah".
- Extend the swab to the back of the oral cavity, being careful not to touch lips, walls of mouth, uvula, or tongue.
- Rubbing the swab over both tonsillar pillars and posterior oropharynx. Avoid touching the tongue, teeth, and gums.
- Place the swab into the provided transport media
- Grasp the top of the swab and bend within the media container until the shaft breaks. If there is no indicator line, bend within the media container until the shaft breaks.
- Break the shaft short enough to fit completly into the media container.
- Replace teh media cap and screw on securely. Label with the OP source
Refrigerated (preferred) - 72 hours
Frozen - 7 days
Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Not detected
Nasopharyngeal (NP) vs oropharyngeal (OP) collections
There is a severe swab shortage. When an NP swab isn’t available, an OP or anterior nares swab is acceptable. The OP swabs can also be used for anterior nares but not for NP (there is no support for using an OP swab as an NP swab).There are some short swabs that can also be used for nares but are too short for OP.
For consistency’s sake and per Mayo’s recommendation for OP as the next step, Allina Health is using OP when NP isn’t available.
Sensitivity
We don’t have a clear answer on sensitivity as there are too many confounding factors. Swabs aren’t the only factor – the viral load is highest for the first few days after symptom onset – swabs collected too early or later in the disease course will have lower sensitivity. This is a confounding factor for published studies that don’t take into account the point in the disease course when the swab was taken.
The most important factor in sampling is to get adequate upper respiratory epithelial cells (where the virus proliferates), and not just secretions.