Prostate cancer gene 3-12479
Test info
PROGENSA PCA3
Prostate cancer gene 3 (PCA3) is strongly expressed in 95% of primary prostate cancer specimens. The PCA3 test is indicated for use in conjunction with other patient information to aid in the decision for repeat biopsy in men age 50 or older who have had one or more previous negative prostate biopsies and for whom a repeat biopsy would be recommended by a urologist based on current standard of care. The PCA3 result provides a risk assessment of a positive biopsy. This assay should not be used for men with atypical small acinar proliferation (ASAP) on their most recent biopsy. Men with ASAP on their most recent biopsy should be treated in accordance with current medical guidelines.
Specimen
Before collection, the patient should undergo an attentive digital rectal exam (three strokes per lobe).
Collect a first-catch (approximately 20 to 30 mL of the initial stream) urine sample into a urine collection cup after DRE has been performed.
- Invert the sample five times to resuspend the cells.
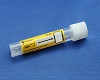
- Using the disposable pipette provided in the kit, transfer 2.5 mL of urine (fill until the fluid level is between the black lines) into the urine specimen transport tube from the Gen-Probe Progensa PCA3 urine specimen transport vial/tube
- Recap the urine specimen transport tube tightly and invert five times to mix.
- Freeze
Frozen (preferred) - 3 months
Refrigerated - 14 days
- Specimen volume less than 2.5 mL
- Incorrect collection kit
- Specimen received after five days
Performance
Target capture, transcription-mediated amplification (TMA) and hybrid protection assay (HPA)
Clinical and Interpretive info
An interpretive report will be provided