COVID-19 Molecular-15064
Test info
2019 Novel Coronavirus (COVID-19), NAA
Coronavirus
CoV
COVID-19
nCoV
SARS-CoV-2
SARS
COVID
Detection of SARS-CoV-2 in patients suspected of viral infection with SARS-CoV-2.
For medical purposes only; not for travel screening.
Excessive mucus is a known inhibitor/interfering substance for molecular tests.
Specimen
Please have patient blow their nose prior to NP and nasal collections. Excessive mucus is known inhibitor/interfering substance for molecular testing.
For Allina Health Clinic Ambulatory patients only:
RespDirect (Enhanced Direct Load Tube)
For all other patients:
For Allina Health Clinic Ambulatory patients:
Nasal collection
Note: Swab included in RespDirect (Enhanced Direct Load Tube) kit is also acceptable for nasopharyngeal collections. See NP collection instructions below.
- Insert swab in one nostril 1/2”-3/4” until swab tip is no longer visible.
- Rotate the swab using moderate pressure along the nostril wall at least 4 times.
- Complete the collection by swabbing the other nostril in the same fashion.
- Uncap the transport media, insert the swab into the media, break swab against the tube at the score line, recap.
For all other patients:
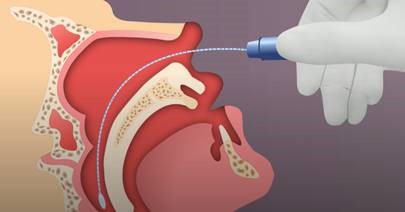
Nasopharyngeal (NP) swab collection:
Use the mini-tip NP swab included in the UTM packaging.
- Tip the patient’s head back.
- Gently insert the NP swab into the nostril parallel to the palate (not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx.
- If any resistance is met in the passageways, do not force the swab; back off and try reinserting it at a different angle, closer to the floor of the nasal canal, or try the other nostril.
- Gently rub and roll the swab for 10-15 seconds while the swab is in contact with the nasopharyngeal wall.
- The CDC recommends leaving the swab in place for several seconds to absorb secretions.
- Slowly remove the swab and place in the transport medium.
- Break the swab shaft so that it fits into the medium container and recap tightly.
- Label the specimen appropriately. Document the source “NP” on the label.
- Request that the patient reapply their mask.
New England Journal of Medicine video on NP swab collection: Nasopharyngeal Swab Collection Video
RespDirect (Enhanced Direct Load Tube) - Allina Health Clinic Ambulatory Patients only
Universal Transport Media
RespDirect (Enhanced Direct Load Tube) - (Allina Health Clinic Ambulatory)
- Refrigerated - 6 days
- Ambient - 6 days
Universal Transport Media (UTM)
- Refrigerated (2-8°C) - 7 days
- Oropharyngeal specimens
- Swabs with cotton tips
- Swabs with wooden shafts
- ESwabs
- Dry swabs
- Swabs with calcium alginate tips
- Leaking specimen(s)
Performance
Outpatient - 12 hours
Nucleic acid amplification test (NAAT)
Clinical and Interpretive info
Not detected
Sensitivity
We don’t have a clear answer on sensitivity as there are too many confounding factors. Swabs aren’t the only factor – the viral load is highest for the first few days after symptom onset – swabs collected too early or later in the disease course will have lower sensitivity. This is a confounding factor for published studies that don’t take into account the point in the disease course when the swab was taken.
The most important factor in sampling is to get adequate upper respiratory epithelial cells (where the virus proliferates), and not just secretions.
Result reporting
Results are reported to applicable state health department based on patient’s address.
Excessive mucus is a known inhibitor/interfering substance for molecular tests. Review specimen tab for patient preparation.