Prostatic specific antigen, total & free-4765
Test info
Prostatic specific antigen
The test method changed on 1/3/2023. If this test has been used for serial monitoring, rebaselining is recommended. Rebaselining consists of 2 measurements, collected 3-6 weeks apart.
The Roche Elecsys CEA assay is an electrochemiluminescence immunoassay "ECLIA" performed on the Roche Cobas "e" immunoassy analyzers.
Values obtained with different assay methods may be different and cannot be used interchangeably.
Specimen
It is recommended to obtain specimens for PSA testing prior to procedures involving manipulation of the prostate.
Immediatley following collection, mix sample by inverting 5 times.
- Allow sample to clot for a minimum of 30 minutes
- Spin within two (2) hours of sample collection
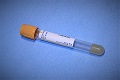
Gold serum separator (SST) tube
Lithium heparin (Li hep) plasma
Immediatley following collection, mix sample by inverting 5 times
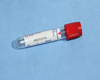
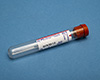
Red:
- Allow sample to clot
- Spin
- Transfer serum to a False bottom plasma/serum transport vial/tube (AHL), labelled as serum, within two (2) hours of sample collection
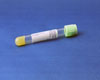
Lt green:
- Spin within two (2) hours of sample collection
Refrigerated (preferred) - 5 days
Frozen - 3 months
Ambient - 8 hours
- Improper labels (unlabeled or mislabeled)
- Hemolysis (some procedures)
- Improper anticoagulant or ratio
- Delay in transport
- Improper storage temperature affecting results
- Improper container
- Leaking container resulting in compromised specimen
- Quantity not sufficient (QNS)
Performance
Electrochemiluminescence immunoassay (ECLIA)
Clinical and Interpretive info
When the total PSA is <4.0 or >10.0, the % Free PSA will not be calculated. The % Free PSA will be reported as "Not Specified"
In patients with total PSA concentrations between 4.0 and 10.0 ng/mL, the Prostate Health Index (PHI) may aid in distinguishing prostate cancer from benign prostate conditions.
Values obtained with different assay methods may be different and cannot be used interchangeably.
Billing
84153