Respiratory panel multiplex PCR-12653
Test info
Respiratory viral panel
RPP
RVP
Viral
Viral panel
- Adenovirus (AdV)
- Bordetella parapertussis
- Bordetella pertussis
- Chlamydia pneumoniae
- Coronavirus (CoV) 229E, HKU1, NL63, OC43
- Human Rhinovirus (HRV)/Enterovirus (EV)
- Human Metapneumovirus (hMPV)
- Influenza A (Flu A) (subtypes H1, H1-2009, and H3)
- Influenza B (Flu B)
- Mycoplasma pneumoniae
- Parainfluenza Virus 1 (PIV1)
- Parainfluenza Virus 2 (PIV2)
- Parainfluenza Virus 3 (PIV3)
- Parainfluenza Virus 4 (PIV4)
- Respiratory syncytial virus (RSV)
- SARS-Cov-2
Triage of in-patients, emergency department/observation patients, and high-risk outpatients (immunocompromised or underlying cardiac/lung conditions) presenting with signs and symptoms of acute respiratory illness. Tests for 17 viral and 3 bacterial respiratory pathogens, however, does NOT detect MERS (Middle Eastern Respiratory Syndrome) or SARS (Severe Acute Respiratory Syndrome).
Ambulatory patients should have targeted testing for respiratory pathogens if indicated.
This assay does not detect MERS (Middle Eastern Respiratory Syndrome) or SARS (Severe Acute Respiratory Syndrome).
The detection of viral and bacterial nucleic acid is dependent upon proper specimen collection, handling, transportation, storage and preparation. Failure to observe proper procedures in any one of these steps can lead to incorrect results. There is a risk of false positive or false negative values resulting from improperly collected, transported or handled specimens.
A negative panel result does not exclude the possibility of viral or bacterial infection. Negative test results may occur from the presence of sequence variants (or mutation) in the region targeted by the assay, the presence of inhibitors, technical error, sample mix-up, an infection caused by an organism not detected by the panel, or lower respiratory tract infection that is not detected by a nasopharyngeal swab specimen. Test results may also be affected by concurrent antiviral/antibacterial therapy or levels of organism in the specimen that are below the limit of detection for the test. Negative results should not be used as the sole basis for diagnosis, treatment, or other patient management decisions.
Specimen
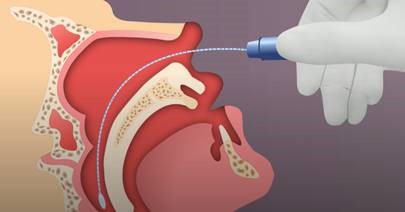
Nasopharyngeal (NP) swab collection:
- Tip the patient’s head back.
- Gently insert the NP swab into the nostril parallel to the palate (not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx.
- If any resistance is met in the passageways, do not force the swab; back off and try reinserting it at a different angle, closer to the floor of the nasal canal, or try the other nostril.
- Gently rub and roll the swab for 10-15 seconds while the swab is in contact with the nasopharyngeal wall.
- The CDC recommends leaving the swab in place for several seconds to absorb secretions.
- Slowly remove the swab and place in the transport medium.
- Break the swab shaft so that it fits into the medium container and recap tightly.
- Label the specimen appropriately. Document the source “NP” on the label.
New England Journal of Medicine video on NP swab collection: Nasopharyngeal Swab Collection Video
BD Universal Viral transport media (UTM)
Viral transport media (VTM), M4, M4RT or M5
Buffered saline (PBS)
Viral transport media (VTM), M4, M4RT or M5
Buffered saline (PBS)
Refrigerated (preferred) - 3 days
Ambient - 4 hours
Frozen (<-15°C) - 30 days
- Improper label (unlabeled or mislabeled)
- Time delay between time of collection and receipt of specimen
- Improper transport container
- Improper transport temperature
- Interfering substances
- Inappropriate specimen type
- Leaking container
Performance
Real time Polymerase Chain Reaction (RT-PCR)
Clinical and Interpretive info
Not detected
Detected: The assay(s) for the organism were POSITIVE.
Not detected: The assay(s) for the organism were NEGATIVE.
Invalid: Failure of assay controls. Unable to determine detected (positive) or not detected (negative) for the target organism(s).
The results of this test should not be used as the sole basis for diagnosis, treatment, or other management decisions.
| Organism (abbreviation) |
Classification (Genome type) |
Season of Highest Incidencea |
Most Commonly Infected Demographic |
|
Adenovirus (AdV) |
Adenovirus (DNA) |
Late winter to early summer |
All ages, immunocompromised |
|
Bordetella parapertussis |
Bacterium (DNA) |
No peak season |
All ages |
|
Bordetella pertussis |
Bacterium (DNA) |
No peak season |
All ages |
|
Chlamydophila pneumoniae |
Bacterium (DNA) |
No peak season |
Older children, young adults, immunocompromised |
|
Coronavirus (CoV) 229E,HKU1, NL63, OC43 |
Coronavirus (RNA) |
Winter, spring |
Children, adults |
|
Enterovirus (EV) |
Picornavirus(RNA) |
Summer, early fall |
All ages |
|
Human Rhinovirus (HRV) |
Picornavirus (RNA) |
Fall, spring |
All ages |
|
Human Metapneumovirus (hMPV) |
Paramyxovirus (RNA) |
Winter, early spring |
Children |
|
Influenza A (Flu A) (subtypes H1, H1-2009, and H3) |
Orthomyxovirus (RNA)
|
Winter |
All ages, 5-20 % of US populationb |
|
Influenza B (Flu B) |
Orthomyxovirus (RNA) |
Winter |
All ages, 5-20 % of US Populationb |
|
Mycoplasma pneumoniae |
Bacterium (DNA) |
Outbreaks most common in summer, outbreak periodicity 4 – 7 years |
Older children, young adults |
|
Parainfluenza Virus 1 (PIV1) |
Paramyxovirus (RNA) |
Fall, periodicity of 1-2 years |
Infants, young children, immunocompromised |
|
Parainfluenza Virus 2 (PIV2) |
Paramyxovirus (RNA) |
Fall, periodicity of 1-2 years |
Infants, young children, immunocompromised |
|
Parainfluenza Virus 3 (PIV3) |
Paramyxovirus (RNA) |
Spring, summer |
Infants, young children, Immunocompromised |
|
Parainfluenza Virus 4 (PIV4) |
Paramyxovirus (RNA) |
Unknown |
All ages |
|
Respiratory Syncytial Virus (RSV) |
Paramyxovirus (RNA) |
Winter, varies by location[ |
Children, older adults
|
|
SARS-CoV-2 |
Coronavirus (RNA) |
Novel pandemic Coronavirus |
All ages |
a Based on North American seasons
b During annual Influenza epidemics, 5-20% of the population is affected with upper respiratory tract infections with rapid onset of fever.
Billing
0202U