COVID/FLU/RSV panel-14423
Test info
- SARS-CoV-2
- Influenza A
- Influenza B
- Respiratory syncytial virus (RSV)
Aiding in diagnosis of common viral pathogens causing severe disease in patients with signs and symptoms of influenza-like-illness (ILI).
- A false negative result may occur if a specimen is improperly collected, transported or handled. False negative results may also occur if inadequate numbers of viral organisms are present in the specimen.
- As with any molecular test, mutations within the target regions could affect primer and/or probe binding resulting in failure to detect the presence of virus.
- Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
- Negative results do not preclude infection and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
Specimen
Please have patient blow their nose prior to NP and nasal collections. Excessive mucus is known inhibitor/interfering substance for molecular testing.
For Allina Health Clinic Ambulatory patients only:
RespDirect (Enhanced Direct Load Tube)
For all other patients:
For Allina Health Clinic Ambulatory patients:
Nasal collection
Note: Swab included in RespDirect (Enhanced Direct Load Tube) kit is also acceptable for nasopharyngeal collections. See NP collection instructions below.
- Insert swab in one nostril 1/2”-3/4” until swab tip is no longer visible.
- Rotate the swab using moderate pressure along the nostril wall at least 4 times.
- Complete the collection by swabbing the other nostril in the same fashion.
- Uncap the transport media, insert the swab into the media, break swab against the tube at the score line, recap.
For all other patients:
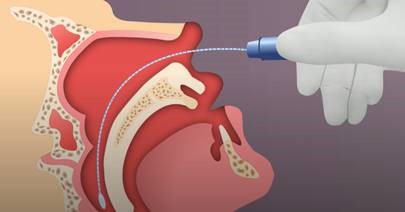
Nasopharyngeal (NP) swab collection:
Use the mini-tip NP swab included in the UTM packaging.
- Tip the patient’s head back.
- Gently insert the NP swab into the nostril parallel to the palate (not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx.
- If any resistance is met in the passageways, do not force the swab; back off and try reinserting it at a different angle, closer to the floor of the nasal canal, or try the other nostril.
- Gently rub and roll the swab for 10-15 seconds while the swab is in contact with the nasopharyngeal wall.
- The CDC recommends leaving the swab in place for several seconds to absorb secretions.
- Slowly remove the swab and place in the transport medium.
- Break the swab shaft so that it fits into the medium container and recap tightly.
- Label the specimen appropriately. Document the source “NP” on the label.
New England Journal of Medicine video on NP swab collection: Nasopharyngeal Swab Collection Video
RespDirect (Enhanced Direct Load Tube) - Allina Health Clinic Ambulatory Patients only
Universal Transport media (UTM)
RespDirect (Enhanced Direct Load Tube) - Allina Health Clinic Ambulatory Patients only
- Refrigerated - 6 days
- Ambient - 6 days
Universal Transport media (UTM)
- Refrigerated (2-8°C) - 7 days
- Improper label (unlabeled or mislabeled)
- Oropharyngeal specimens
- Specimens types, swabs, and/or collection containers other than listed above.
- Specimens that do not meet listed transport and stability above.
- Leaking container
Performance
Central, Mercy and United
STAT - 2.5 hours
Realt time nucleic acid amplification (NAAT)
Clinical and Interpretive info
Negative
Excessive mucus is a known inhibitor/interfering substance for molecular tests. Review specimen tab for patient preparation.