Follicle stimulating hormone by IHC-12376 - Technical only, 12379 - Technical & interpretation
Test info
Follicle stimulating hormone by IHC
12376 - Technical only, 12379 - Technical & interpretation
LAB12376
LAB12379
LAB12379
FSH
All IHC stains will include a positive control tissue
- IHCstaining for the presence of pituitary hormones helps distinguish pituitary adenomas and the rare primary pituitary carcinomas from other tumors occurring in the region of the sella (such as craniopharyngioma, metastatic carcinoma, meningioma, lymphoma, leukemia and plasmacytoma), none of which contain or produce pituitary hormones
- Positive staining for specific pituitary hormones can help classify pituitary adenomas into subclasses based on the corresponding cell type present
- Negative staining for pituitary hormones does not rule out a pituitary adenoma since some contain none of the known adenohypophyseal hormones
- Gonadotrophic cell adenomas account for 10-15% of all pituitary adenomas. They usually stain for both LH and FSH
Specimen
Tissue
Submit a formalin-fixed, paraffin-embedded tissue
Formalin-fixed, paraffin-embedded (FFPE) tissue block
FFPE tissue section mounted on a charged, unstained slide
Ambient (preferred)
- Unlabeled/mislabeled block
- Insufficient tissue
- Slides broken beyond repair
Performance
AHL - Immunohistochemistry
Mo - Fr
1 - 2 days
Immunohistochemical staining and microscopic examination
Clinical and Interpretive info
If requested, an interpretive report will be provided
Specifications
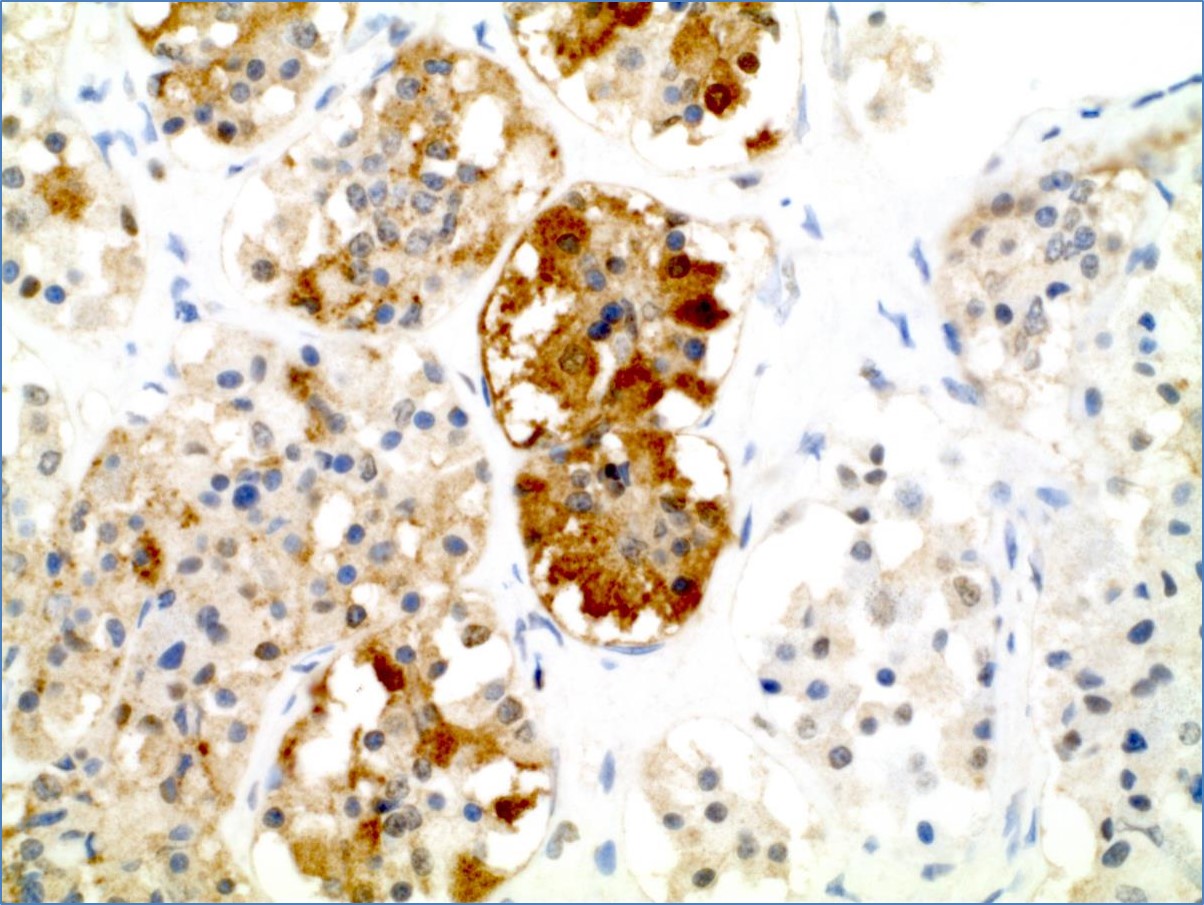
- This antibody reacts with follicle stimulating hormone. It recognizes the gonadotrophic cells of the pituitary gland
- In the normal pituitary gland the gonadotrophs comprise about 10% of the cells and are scattered throughout the adenohypophysis with some concentration in the posterolateral region
- In some gonadotrophs, FSH and LH can be demonstrated in the same cell, but most gonadotrophs contain only one of the hormones
Staining pattern
- Cytoplasmic based staining
Billing
88342 - 1st stain
88341 - each additional stain
88341 - each additional stain
Tracking
10/30/2017
01/31/2024
01/31/2022