Toxoplasma gondii DNA Detection, Ocular/Vitreous Fluid-994
Test info
Toxoplasma gondii DNA Detection, Ocular/Vitreous Fluid
994
LAB994
MSO
Toxoplasma gondii DNA Detection, Eye Fluid
Specimen
Ocular/vitreous fluid, undiluted
1.0 mL
To avoid unnecessary aliquoting/transferring of sample to minimize volume loss, low-volume samples ocular specimens can be left in a properly packaged syringe.
- Remove all sharps
- Place a screw-on cap at end of syringe and secure cap by wrapping with Parafilm
- Leave an airspace in the syringe to accommodate expansion upon freezing
- Stabilize the plunger by wrapping with Parafilm (no adhesive tape)
- Do not place pressure on the plunger
- Place the parafilm secured syringe in a sealable specimen bag
- Freeze samples for PCR testing within two hours of collection
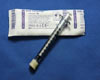
Syringe (1mL)
Syringe - ocular fluid (1 mL)
- Needle removed
- Securely capped to prevent inadvertent plunging during transport and resulting loss of specimen
For specimens submitted by Eye Surgeons only:
- U of Washington request form
- The following items are required:
- Patient name
- Patient DOB
- Ordering physician
- Ordering physician phone number
- Specimen source
- Date and time collected
- ICD/Diagnosis
- The following items are required:
Frozen (preferred)
Ambient – 2 hours
Refrigerated - 8 hours
- Diluted sample
Performance
U of Washington (TOXDNA): R-NX
4 days
DNA extraction, nucleic acid purification, polymerase chain reaction (PCR), sequencing
Clinical and Interpretive info
See report
Billing
This test may require preauthorization from the insurance provider. Check the payer guidelines and, if needed, obtain the pre-authorization prior to sample collection.
87798
All charges for this test will be billed back to the requesting site
17028777
19028777
Tracking
11/19/2020
01/08/2024
01/08/2024