Ocular/Vitreous Fluid Viral PCR Quant Panel (CMV, HSV, VZV)
Ocular/Vitreous Fluid Viral PCR Quant Panel (CMV, HSV, VZV)-994
Ocular/Vitreous Fluid Viral PCR Quant Panel (CMV, HSV, VZV)
994
LAB994
MSO
Eye fluid viral PCR quant panel (CMV, HSV, VZV)
Viral Quant Panel, Eye Fluid
Viral Quant Panel, Eye Fluid
- Cytomegalovirus
- Herpes simplex virus
- Varicella zoster virus
Eye/vitreous fluid, undiluted or diluted
To avoid unnecessary aliquoting/transferring of sample to minimize volume loss, low-volume samples ocular specimens can be left in a properly packaged syringe.
- Remove all sharps
- Place a screw-on cap at end of syringe and secure cap by wrapping with Parafilm
- Leave an airspace in the syringe to accommodate expansion upon freezing
- Stabilize the plunger by wrapping with Parafilm (no adhesive tape)
- Do not place pressure on the plunger
- Place the parafilm secured syringe in a sealable specimen bag
- Freeze samples for PCR testing within two hours of collection
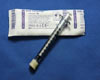
Syringe (1mL)
Syringe - ocular fluid (1 mL)
- Needle removed
- Securely capped to prevent inadvertent plunging during transport and resulting loss of specimen
- U of Washington request form
- The following items are required:
- Patient name
- Patient DOB
- Ordering physician
- Ordering physician phone number
- Specimen source
- Date and time collected
- ICD/Diagnosis
- Prioritization of testing
- The following items are required:
Frozen (preferred)
Ambient – 2 hours
Refrigerated – 48 hours
U of Washington (EYEVQP): R-NX
Twice per week
4 days
Real Time PCR (RT-PCR)
See report
87497
87530 x 2
87799
87530 x 2
87799
All charges for this test will be billed back to the requesting site
11/20/2020
01/08/2024
01/08/2024