Solid tumor targeted NGS panel
Solid tumor targeted NGS panel-Pathology add-on
Next Generation Sequencing
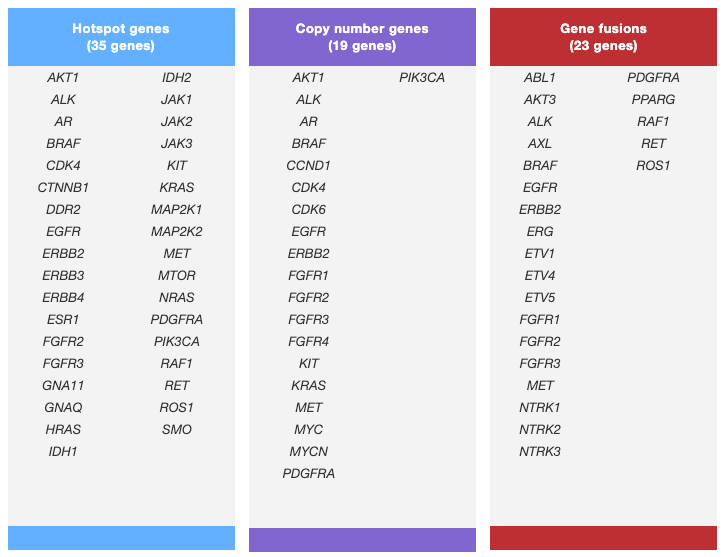
Oncomine focus assay
52 genes
Next Generation Sequencing (NGS) for the detection of alterations in solid tumors is critical for cancer care as these findings can direct the optimal use of targeted therapies for patients.
If blocks more than 5 years old are submitted for testing, extraction will be attempted, but if insufficient RNA/DNA is retrieved, testing will be cancelled
Formalin-fixed paraffin-embedded (FFPE) tissue block
Cytology slide
Molecular Medicare billing request
Hospital clients submitting a request for this assay on an outpatient with Medicare should complete and submit a Molecular Medicare billing request form to notify us of the need for Allina Health Laboratory to bill insurance.
Ambient
- Improper label (unlabeled or mislabeled)
- Improper storage
- Interfering substances
- Slides broken beyond repair
Next Generation Sequencing
Hospital clients submitting a request for this assay on an outpatient with Medicare should complete and submit a Molecular Medicare billing request form to notify us of the need for Allina Health Laboratory to bill insurance.