Mpox (Orthopoxvirus), DNA, PCR
Mpox (Orthopoxvirus), DNA, PCR-14585
Orthopox virus
Monkeypox
Vesicular specimens collected from persons infected with a non-variola Orthopoxvirus (such as vaccinia, monkeypox, or cowpox) are expected to produce a positive result with this assay. Although this assay does not differentiate vaccinia or monkeypox virus from cowpox, camelpox, ectomelia or gerbilpox virus, a positive result with this assay in the United States is most likely due to monkeypox virus or vaccinia virus; however, potential exposure to other Orthopoxviruses should be considered.
- A false negative result may occur if a sample is improperly collected, transported or handled. False negative results may occur if the quantity of viral DNA present in the sample is below the limit of detection for the assay.
- An inconclusive result may be returned if the lesion is not adequately sampled.
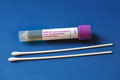
- Collect one swab from the same lesion, or from another lesion with similar appearance.
- Vigorously swab or brush the base of the lesion with a sterile dry polyester, rayon or Dacron swab.
- Insert swab into the tube containing UTM.
- Carefully break the swabs at the scoreline and tightly close the sample.
If multiple lesions with differing appearances are present, consider submitting additional collections, as described above, for each lesion type.
Copan UTM-RT transport (purple cap)
- Vigorously swab or brush the base of the lesion with a sterile dry polyester, rayon or Dacron swab.
- Collect a second swab from the same lesion.
- Insert swab into a sterile vial.
Refrigerated (preferred)– 7 days
Frozen - 30 days
- Improperly submitted specimen
- Grossly leaking samples
- Swabs submitted in non-validated transport media, including 0.9% saline or liquid Amies (E-Swabs)
- Swab specimens submitted in aerobic or anaerobic transport devices
Polymerase chain reaction (PCR)
Not detected