Myeloid NGS
Myeloid NGS-14519
Myeloproliferative neoplasms
Myelodysplastic syndromes
MPN
MDS
Myeloid leukemias
AML
NGS
FLT3
IDH1
IDH2
TP53
NPM1
CEBPA
RUNX1
KIT
DNMT3A
KRAS
ASXL1
BCR/ABL
JAK2
CALR
MPL
MYD88
SF3B1
One of the customized panels can be selected below including any single gene add ons in the following tables.
This Myeloid NGS panel includes 71 genes. The panel consists a DNA component designed to detect mutations as well as an RNA component designed to detect gene fusions. This test is intended for the molecular evaluation of myeloid neoplasms, including myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), myelodysplastic/myeloproliferative neoplasms, and myeloid leukemias (AML) among others. The detection of molecular alterations described in these neoplasms can be useful in their diagnosis and classification. In addition, the pattern of particular alterations can be prognostically informative and can have implications for the use of both targeted and conventional therapies. This NGS panel will serve to consolidate several single gene assays into a single test. Preset gene panels are available for order as well as individual genes if desired.
Immediately following collection, mix sample by inverting 8 - 10 times to prevent clotting.
Submit unspun
Sodium citrate (Na cit) whole blood
Bone marrow in EDTA
Molecular Medicare billing request
Hospital clients submitting a request for this assay on an outpatient with Medicare should complete and submit a Molecular Medicare billing request form to notify us of the need for Allina Health Laboratory to bill insurance.
Refrigerated (preferred) - 7 days
Ambient - 4 days
Do not freeze
- Heparin collection
- Frozen specimens
Next Generation Sequencing
One of the customized panels can be selected below including any single gene add ons in the following tables.
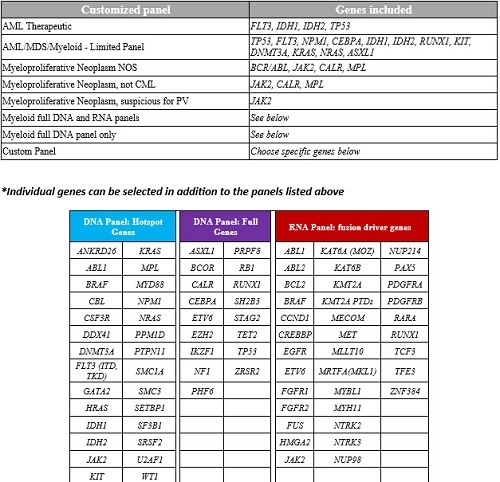
| Customized panel | Genes included |
| AML Therapeutic | FLT3, IDH1, IDH2, TP53 |
| AML/MDS/Myeloid - Limited Panel | TP53, FLT3, NPM1, CEBPA, IDH1, IDH2, RUNX1, KIT, DNMT3A, KRAS, NRAS, ASXL1 |
| Myeloproliferative Neoplasm NOS | BCR/ABL, JAK2, CALR, MPL |
| Myeloproliferative Neoplasm, not CML | JAK2, CALR, MPL |
| Myeloproliferative Neoplasm, suspicious for PV | JAK2 |
| Myeloid full DNA and RNA panels | See below |
| Myeloid full DNA panel only | See below |
| Custom Panel | Choose specific genes below |
*Individual genes can be selected in addition to the panels listed above
| DNA Panel: Hotspot Genes | DNA Panel: Full Genes | RNA Panel: fusion driver genes | ||||||
| ANKRD26 | KRAS | ASXL1 | PRPF8 | ABL1 | KAT6A (MOZ) | NUP214 | ||
| ABL1 | MPL | BCOR | RB1 | ABL2 | KAT6B | PAX5 | ||
| BRAF | MYD88 | CALR | RUNX1 | BCL2 | KMT2A | PDGFRA | ||
| CBL | NPM1 | CEBPA | SH2B3 | BRAF | KMT2A PTDs | PDGFRB | ||
| CSF3R | NRAS | ETV6 | STAG2 | CCND1 | MECOM | RARA | ||
| DDX41 | PPM1D | EZH2 | TET2 | CREBBP | MET | RUNX1 | ||
| DNMT3A | PTPN11 | IKZF1 | TP53 | EGFR | MLLT10 | TCF3 | ||
| FLT3 (ITD, TKD) | SMC1A | NF1 | ZRSR2 | ETV6 | MRTFA(MKL1) | TFE3 | ||
| GATA2 | SMC3 | PHF6 | FGFR1 | MYBL1 | ZNF384 | |||
| HRAS | SETBP1 | FGFR2 | MYH11 | |||||
| IDH1 | SF3B1 | FUS | NTRK2 | |||||
| IDH2 | SRSF2 | HMGA2 | NTRK3 | |||||
| JAK2 | U2AF1 | JAK2 | NUP98 | |||||
| KIT | WT1 | |||||||
AML/MDS/Myeloid - Limited Panel: 81450
Myeloproliferative Neoplasm NOS: 81450
Myeloproliferative Neoplasm, not CML: 81279, 81219, 81338
Myeloproliferative Neoplasm, suspicious for PV: 81279
DNA Panel: Hotspot Genes: 81450
DNA Panel: Full Genes: 81450
RNA Panel: fusion driver genes: 81450
MYELOID NGS JAK2 EXON 12-15: 81279
MYELOID NGS CALR: 81219
MYELOID NGS MPL: 81338
MYELOID NGS IDH1: 81120
MYELOID NGS IDH2: 81121
MYELOID NGS SF3B1:81347
MYELOID NGS MYD88: 81305
MYELOID NGS BRAF: 81210
MYELOID NGS KIT: 81272
MYELOID NGS TP53: 81352
MYELOID NGS FLT3 ITD: 81245
MYELOID NGS FLT3 TKD: 81246
MYELOID NGS NPM1: 81310
MYELOID NGS CEBPA: 81218
MYELOID NGS RARA: 81315
MYELOID NGS BCR/ABL: 81206
MYELOID NGS KRAS CODONS 12 AND 13: 81275
MYELOID NGS KRAS CODONS 61 AND 146: 81276
MYELOID NGS NRAS: 81311
Codes listed are associated with most common orders and is not inclusive of all potential orders
Hospital clients submitting a request for this assay on an outpatient with Medicare should complete and submit a Molecular Medicare billing request form to notify us of the need for Allina Health Laboratory to bill insurance.