Epstein-Barr Virus (EBV) Quantitative by PCR, Ocular/Vitreous Fluid
Epstein-Barr Virus (EBV) Quantitative by PCR, Ocular/Vitreous Fluid-994
Epstein-Barr Virus (EBV) Quantitative by PCR, Ocular/Vitreous Fluid
994
LAB994
MSO
Epstein Barr Virus (EBV) Quantitative by PCR, Eye Fluid
Ocular/vitreous fluid, undiluted or diluted
1.0 mL
0.5 mL
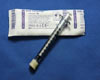
To avoid unnecessary aliquoting/transferring of sample to minimize volume loss, low-volume samples ocular specimens can be left in a properly packaged syringe.
- Remove all sharps
- Place a screw-on cap at end of syringe and secure cap by wrapping with Parafilm
- Leave an airspace in the syringe to accommodate expansion upon freezing
- Stabilize the plunger by wrapping with Parafilm (no adhesive tape)
- Do not place pressure on the plunger
- Place the parafilm secured syringe in a sealable specimen bag
- Freeze samples for PCR testing within two hours of collection
Syringe (1mL)
- The following items are required:
- Patient name
- Patient DOB
- Ordering physician
- Ordering physician phone number
- Specimen source
- Date and time collected
- ICD/Diagnosis
Frozen (preferred)
Refrigerated - 48 hours
U of Washington (EBVQ): R-NX
Twice per week
7 days
Polymerase Chain Reaction (PCR)
See report
87799
All charges for this test will be billed back to the requesting site
11/25/2020
01/08/2024
01/08/2024